Hydrogen has an average atomic mass of 1.00794 amu. Oxygen atoms have an average mass of 15.9994 amu. The average mass of a molecule of H 2 O equals (1.00794) (2) + 15.9994 = 18.01528 amu, equivalent to 18.01528 g/mol. Average atomic masses listed by IUPAC are based on a study of experimental results. Bromine has two isotopes, 79 Br and 81 Br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69% and 49.31%) were determined in earlier experiments. Calculate the average atomic mass of Br based on these experiments. To calculate the average mass, first convert the percentages into fractions (divide them by 100). Then, calculate the mass numbers. The chlorine isotope with 18 neutrons has an abundance of 0.7577 and a mass number of 35 amu. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together.
- Calculating Average Atomic Mass Quiz
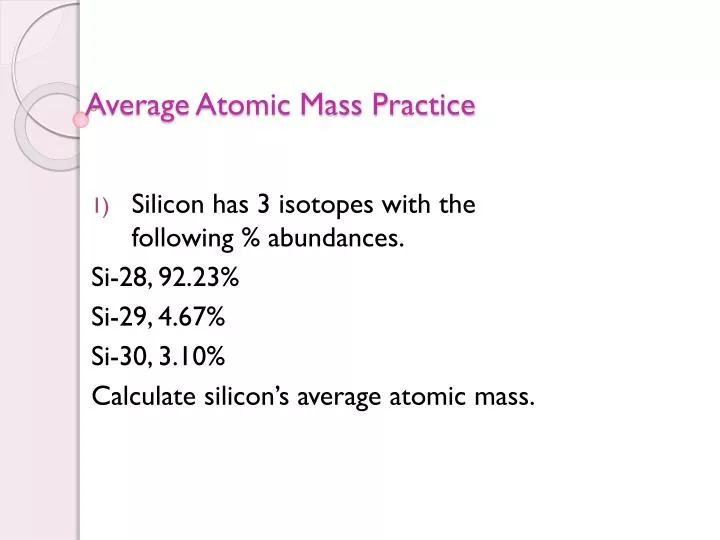
- Calculating Average Atomic Mass From Percent Abundance
- Calculating Average Atomic Mass
- Calculating Average Atomic Mass Worksheet Pdf
The average atomic mass of an element is the sum of the masses of its isotopes, each multiplied by its natural abundance.
Learning Objectives
- Calculate the average atomic mass of an element given its isotopes and their natural abundance
Key Points
- An element can have differing numbers of neutrons in its nucleus, but it always has the same number of protons. The versions of an element with different neutrons have different masses and are called isotopes.
- The average atomic mass for an element is calculated by summing the masses of the element’s isotopes, each multiplied by its natural abundance on Earth.
- When doing any mass calculations involving elements or compounds, always use average atomic mass, which can be found on the periodic table.
Key Terms
- mass number: The total number of protons and neutrons in an atomic nucleus.
- natural abundance: The abundance of a particular isotope naturally found on the planet.
- average atomic mass: The mass calculated by summing the masses of an element’s isotopes, each multiplied by its natural abundance on Earth.
The atomic number of an element defines the element’s identity and signifies the number of protons in the nucleus of one atom. For example, the element hydrogen (the lightest element) will always have one proton in its nucleus. The element helium will always have two protons in its nucleus.
Isotopes
Atoms of the same element can, however, have differing numbers of neutrons in their nucleus. For example, stable helium atoms exist that contain either one or two neutrons, but both atoms have two protons. These different types of helium atoms have different masses (3 or 4 atomic mass units ), and they are called isotopes. For any given isotope, the sum of the numbers of protons and neutrons in the nucleus is called the mass number. This is because each proton and each neutron weigh one atomic mass unit (amu). By adding together the number of protons and neutrons and multiplying by 1 amu, you can calculate the mass of the atom. All elements exist as a collection of isotopes. The word ‘isotope’ comes from the Greek ‘isos’ (meaning ‘same’) and ‘topes’ (meaning ‘place’) because the elements can occupy the same place on the periodic table while being different in subatomic construction.
Calculating Average Atomic Mass
The average atomic mass of an element is the sum of the masses of its isotopes, each multiplied by its natural abundance (the decimal associated with percent of atoms of that element that are of a given isotope).
Average atomic mass = f1M1 + f2M2 +… + fnMnwhere f is the fraction representing the natural abundance of the isotope and M is the mass number (weight) of the isotope.
Calculating Average Atomic Mass Quiz
The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. When data are available regarding the natural abundance of various isotopes of an element, it is simple to calculate the average atomic mass.
- For helium, there is approximately one isotope of Helium-3 for every million isotopes of Helium-4; therefore, the average atomic mass is very close to 4 amu (4.002602 amu).
- Chlorine consists of two major isotopes, one with 18 neutrons (75.77 percent of natural chlorine atoms) and one with 20 neutrons (24.23 percent of natural chlorine atoms). The atomic number of chlorine is 17 (it has 17 protons in its nucleus).
To calculate the average mass, first convert the percentages into fractions (divide them by 100). Then, calculate the mass numbers. The chlorine isotope with 18 neutrons has an abundance of 0.7577 and a mass number of 35 amu. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together.
Average atomic mass of chlorine = (0.7577 ⋅⋅ 35 amu) + (0.2423 ⋅⋅ 37 amu) = 35.48 amu
Another example is to calculate the atomic mass of boron (B), which has two isotopes: B-10 with 19.9% natural abundance, and B-11 with 80.1% abundance. Therefore,
Average atomic mass of boron = (0.199⋅⋅10 amu) + (0.801⋅⋅11 amu) = 10.80 amu
Whenever we do mass calculations involving elements or compounds (combinations of elements), we always use average atomic masses.
LICENSES AND ATTRIBUTIONS Ios google contacts sync.
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- ionic bond. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/ionic_bond. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Biology. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44390/latest..ol11448/latest. License: CC BY: Attribution
- Biochemistry/Metabolism and energy. Provided by: Wikibooks. Located at: en.wikibooks.org/wiki/Biochem..%23Ionic_bonds. License: CC BY-SA: Attribution-ShareAlike
- ion. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/ion. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Atoms, Isotopes, Ions, and Molecules: The Building Blocks. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44390/latest..e_02_01_10.jpg. License: CC BY: Attribution
- OpenStax College, Biology. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44390/latest..ol11448/latest. License: CC BY: Attribution
- Biochemistry/Metabolism and energy. Provided by: Wikibooks. Located at: en.wikibooks.org/wiki/Biochem..%23Ionic_bonds. License: CC BY-SA: Attribution-ShareAlike
- dipole. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/dipole. License: CC BY-SA: Attribution-ShareAlike
- covalent bond. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/covalent_bond. License: CC BY-SA: Attribution-ShareAlike
- hydrogen bond. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/hydrogen_bond. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Atoms, Isotopes, Ions, and Molecules: The Building Blocks. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44390/latest..e_02_01_10.jpg. License: CC BY: Attribution
- OpenStax College, Atoms, Isotopes, Ions, and Molecules: The Building Blocks. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44390/latest..e_02_01_11.jpg. License: CC BY: Attribution
- ATP structure. Provided by: Wikimedia. Located at: commons.wikimedia.org/wiki/Fi.._structure.svg. License: Public Domain: No Known Copyright
- Hydrogen bond. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Hydrogen_bond. License: CC BY-SA: Attribution-ShareAlike
- electronegativity. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/electronegativity. License: CC BY-SA: Attribution-ShareAlike
- intermolecular. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/intermolecular. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Atoms, Isotopes, Ions, and Molecules: The Building Blocks. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44390/latest..e_02_01_10.jpg. License: CC BY: Attribution
- OpenStax College, Atoms, Isotopes, Ions, and Molecules: The Building Blocks. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44390/latest..e_02_01_11.jpg. License: CC BY: Attribution
- ATP structure. Provided by: Wikimedia. Located at: commons.wikimedia.org/wiki/Fi.._structure.svg. License: Public Domain: No Known Copyright
- File:3D model hydrogen bonds in water.svg - Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: en.Wikipedia.org/w/index.php?..ter.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- Water drops on green leaf. Provided by: Wikimedia Commons. Located at: commons.wikimedia.org/wiki/Fi..green_leaf.jpg. License: CC BY-SA: Attribution-ShareAlike
- Avogadro's number and the mole. Provided by: Steve Lower's Website. Located at: http://www.chem1.com/acad/webtext/intro/int-2.html#SEC2. License: CC BY-SA: Attribution-ShareAlike
- Mole (unit). Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Mole_(unit). License: CC BY-SA: Attribution-ShareAlike
- Avogadro constant. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Avogadro_constant. License: CC BY-SA: Attribution-ShareAlike
- mole. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/mole. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Atoms, Isotopes, Ions, and Molecules: The Building Blocks. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44390/latest..e_02_01_10.jpg. License: CC BY: Attribution
- OpenStax College, Atoms, Isotopes, Ions, and Molecules: The Building Blocks. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44390/latest..e_02_01_11.jpg. License: CC BY: Attribution
- ATP structure. Provided by: Wikimedia. Located at: commons.wikimedia.org/wiki/Fi.._structure.svg. License: Public Domain: No Known Copyright
- File:3D model hydrogen bonds in water.svg - Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: en.Wikipedia.org/w/index.php?..ter.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- Water drops on green leaf. Provided by: Wikimedia Commons. Located at: commons.wikimedia.org/wiki/Fi..green_leaf.jpg. License: CC BY-SA: Attribution-ShareAlike
- The Mole, Avogadro. Located at: http://www.youtube.com/watch?v=TqDqLmwWx3A. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Avogadro Amedeo. Provided by: Wikimedia. Located at: en.Wikipedia.org/wiki/Avogadr..dro_Amedeo.jpg. License: Public Domain: No Known Copyright
- Introductory Chemistry Online/Measurements and Atomic Structure. Provided by: Wikibooks. Located at: en.wikibooks.org/wiki/Introdu..omic_Structure. License: CC BY-SA: Attribution-ShareAlike
- Atomic mass. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Atomic_mass. License: CC BY-SA: Attribution-ShareAlike
- Average atomic mass. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Average_atomic_mass. License: CC BY-SA: Attribution-ShareAlike
- natural abundance. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/natural%20abundance. License: CC BY-SA: Attribution-ShareAlike
- Boundless. Provided by: Boundless Learning. Located at: www.boundless.com//biology/de..atomic-mass--2. License: CC BY-SA: Attribution-ShareAlike
- isotope. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/isotope. License: CC BY-SA: Attribution-ShareAlike
- mass number. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/mass_number. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Atoms, Isotopes, Ions, and Molecules: The Building Blocks. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44390/latest..e_02_01_10.jpg. License: CC BY: Attribution
- OpenStax College, Atoms, Isotopes, Ions, and Molecules: The Building Blocks. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44390/latest..e_02_01_11.jpg. License: CC BY: Attribution
- ATP structure. Provided by: Wikimedia. Located at: commons.wikimedia.org/wiki/File:ATP_structure.svg. License: Public Domain: No Known Copyright
- File:3D model hydrogen bonds in water.svg - Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: en.Wikipedia.org/w/index.php?title=File:3D_model_hydrogen_bonds_in_water.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- Water drops on green leaf. Provided by: Wikimedia Commons. Located at: commons.wikimedia.org/wiki/Fi..green_leaf.jpg. License: CC BY-SA: Attribution-ShareAlike
- The Mole, Avogadro. Located at: http://www.youtube.com/watch?v=TqDqLmwWx3A. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Avogadro Amedeo. Provided by: Wikimedia. Located at: en.Wikipedia.org/wiki/Avogadr..dro_Amedeo.jpg. License: Public Domain: No Known Copyright
- File:Stylised Lithium Atom.svg - Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: en.Wikipedia.org/w/index.php?..tom.svg&page=1. License: Public Domain: No Known Copyright
Calculate the average atomic weight when given isotopic weights and abundances
Fifteen Examples
To do these problems you need some information: the exact atomic weight for each naturally-occuring stable isotope and its percent abundance. These values can be looked up in a standard reference book such as the 'Handbook of Chemistry and Physics.' The unit associated with the answer can be either amu or g/mol, depending on the context of the question. If it is not clear from the context that g/mol is the desired answer, go with amu (which means atomic mass unit).
This problem can also be reversed, as in having to calculate the isotopic abundances when given the atomic weight and isotopic weights. Study the tutorial below and then look at the problems done in the reverse direction.
Example #1: Carbon
| mass number | exact weight | percent abundance |
| 12 | 12.000000 | 98.90 |
| 13 | 13.003355 | 1.10 |
To calculate the average atomic weight, each exact atomic weight is multiplied by its percent abundance (expressed as a decimal). Then, add the results together and round off to an appropriate number of significant figures.
This is the solution for carbon:
(12.000000) (0.9890) + (13.003355) (0.0110) = 12.011 amu
Example #2: Nitrogen
| mass number | exact weight | percent abundance |
| 14 | 14.003074 | 99.63 |
| 15 | 15.000108 | 0.37 |
This is the solution for nitrogen:
(14.003074) (0.9963) + (15.000108) (0.0037) = 14.007 amu
| Example #3: Chlorine | Example #4: Silicon | ||||
| mass number | exact weight | percent abundance | mass number | exact weight | percent abundance |
| 35 | 34.968852 | 75.77 | 28 | 27.976927 | 92.23 |
| 37 | 36.965903 | 24.23 | 29 | 28.976495 | 4.67 |
| 30 | 29.973770 | 3.10 | |||
| The answer for chlorine: 35.453 | The answer for silicon: 28.086 | ||||
This type of calculation can be done in reverse, where the isotopic abundances can be calculated knowing the average atomic weight. Go to tutorial on reverse direction.
Example #5: In a sample of 400 lithium atoms, it is found that 30 atoms are lithium-6 (6.015 g/mol) and 370 atoms are lithium-7 (7.016 g/mol). Calculate the average atomic mass of lithium.
Solution:
1) Calculate the percent abundance for each isotope:
Li-6: 30/400 = 0.075
Li-7: 370/400 = 0.925
2) Calculate the average atomic weight:
x = (6.015) (0.075) + (7.016) (0.925)x = 6.94 g/mol
Example #6: A sample of element X contains 100 atoms with a mass of 12.00 and 10 atoms with a mass of 14.00. Calculate the average atomic mass (in amu) of element X.
Solution:
1) Calculate the percent abundance for each isotope:
X-12: 100/110 = 0.909
X-14: 10/110 = 0.091
2) Calculate the average atomic weight:
x = (12.00) (0.909) + (14.00) (0.091)x = 12.18 amu (to four sig figs)
3) Here's another way:
100 atoms with mass 12 = total atom mass of 120010 atoms with mass 14 = total atom mass of 140
1200 + 140 = 1340 (total mass of all atoms)
Total number of atoms = 100 + 10 = 110
1340/110 = 12.18 amu
The first way is the standard technique for solving this type of problem. That's because we do not generally know the specific number of atoms in a given sample. More commonly, we know the percent abundances, which is different from the specific number of atoms in a sample.
Example #7: Boron has an atomic mass of 10.81 amu according to the periodic table. However, no single atom of boron has a mass of 10.81 amu. How can you explain this difference?
Solution:
10.81 amu is an average, specifically a weighted average. It turns out that there are two stable isotopes of boron: boron-10 and boron-11.
Neither isotope weighs 10.81 amu, but you can arrive at 10.81 amu like this:x = (10.013) (0.199) + (11.009) (0.801)x = 1.99 + 8.82 = 10.81
Example #8: Copper occurs naturally as Cu-63 and Cu-65. Which isotope is more abundant?
Solution:
Look up the atomic weight of copper: 63.546 amuSince our average value is closer to 63 than to 65, we concude that Cu-63 is the more abundant isotope.
Example #9: Copper has two naturally occuring isotopes. Cu-63 has an atomic mass of 62.9296 amu and an abundance of 69.15%. What is the atomic mass of the second isotope? What is its nuclear symbol?
Calculating Average Atomic Mass From Percent Abundance
Solution:
1) Look up the atomic weight of copper:
63.546 amu

2) Set up the following and solve:
(62.9296) (0.6915) + (x) (0.3085) = 63.54643.5158 + 0.3085x = 63.546
0.3085x = 20.0302
x = 64.9277 amu
3) The nuclear symbol is:
4) You might see this
29-Cu-65This is used in situations, such as the Internet, where the subscript/superscript notation cannot be reproduced. You might also see this:
65/29Cu
Example #10: Naturally occurring iodine has an atomic mass of 126.9045. A 12.3849 g sample of iodine is accidentally contaminated with 1.0007 g of I-129, a synthetic radioisotope of iodine used in the treatment of certain diseases of the thyroid gland. The mass of I-129 is 128.9050 amu.Find the apparent 'atomic mass' of the contaminated iodine.
Solution:
1) Calculate mass of contaminated sample:
12.3849 g + 1.0007g = 13.3856 g
2) Calculate percent abundances of (a) natural iodine and (b) I-129 in the contaminated sample:
(a) 12.3849 g / 13.3856 g = 0.92524
(b) 1.0007 g / 13.3856 g = 0.07476
3) Calculate 'atomic mass' of contaminated sample:
(126.9045) (0.92524) + (128.9050) (0.07476) = xx = 127.0540 amu
Example #11: Neon has two major isotopes, Neon-20 and Neon-22. Out of every 250 neon atoms, 225 will be Neon-20 (19.992 g/mol), and 25 will be Neon-22 (21.991 g/mol). What is the average atomic mass of neon?
Solution:
1) Determine the percent abundances (but leave as a decimal):
Ne-20 ---> 225 / 250 = 0.90Ne-22 ---> 25 / 250 = 0.10
The last value can also be done by subtraction, in this case 1 - 0.9 = 0.1
2) Calculate the average atomic weight:
(19.992) (0.90) + (21.991) (0.10) = 20.19
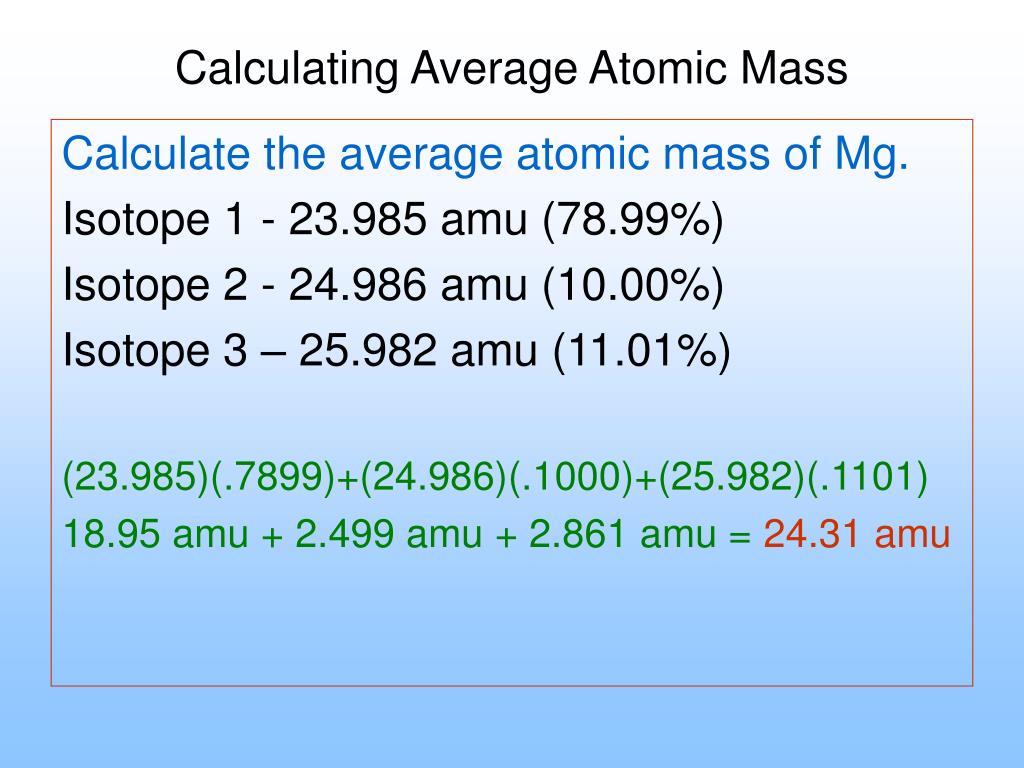
Example #12: Calculate the average atomic weight for magnesium:
| mass number | exact weight | percent abundance |
| 24 | 23.985042 | 78.99 |
| 25 | 24.985837 | 10.00 |
| 26 | 25.982593 | 11.01 |
The answer? Find magnesium on the periodic table:
Remember that the above is the method by which the average atomic weight for the element is computed. No one single atom of the element has the given atomic weight because the atomic weight of the element is an average, specifically called a 'weighted' average. Given that, here is a question you could be asked.
Example #13: Silver has an atomic mass of 107.868 amu. Does any atom of any isotope of silver have a mass of 107.868 amu? Explain why or why not.
Solution:
The specific question is about silver, but it could be any element. The answer, of course, is no. The atomic weight of silver is a weighted average. Silver is not composed of atoms each of which weighs 107.868.
Example #14: Given that the average atomic mass of hydrogen in nature is 1.0079, what does that tell you about the percent composition of H-1 and H-2 in nature?
Solution:

It tells you that the proportion of H-1 is much much greater than the proportion of H-2 in nature.
Example #15: The relative atomic mass of neon is 20.18 It consists of three isotopes with the masses of 20, 21and 22. It consists of 90.5% of Ne-20. Determine the percent abundances of the other two isotopes.
Solution:
1) Let y% be the relative abundance of Ne-21.
2) Then, the relative abundance of Ne-22 is:
(100 − 90.5 − y)% = (9.5 − y)%
3) Relative atomic mass of Ne (note use of decimal abundances, not percent abundances):
(20) (0.905) + (21) (y) + (22) (0.095 − y) = 20.18Calculating Average Atomic Mass
18.10 + 21y + 2.09 - 22y = 20.18
y = 0.01
Navigation; Jump to operand: Enter: Jump in new window + Jump to previous position: Esc: Jump to Next position + Jump to address: G: Jump by name +L: Jump to function +P: Jump to segment +S: Jump to segment register +G: Jump to problem +Q: Jump to cross reference +X: Jump to xref to operand: X: Jump to entry point +E: Mark Position +M. IDA Pro for Static Code Analysis Text search Alt+t Show the operand as a character r Insert repeatable comment; Follow jump or call in view Enter. Creative Commons v3 “Attribution” License for this cheat sheet version 2.0. Title: Malware Analysis and Reverse-Engineering Cheat Sheet Author.  Datarescue Interactive Disassembler (IDA) Pro Quick Reference Sheet (Open Subviews Names Shift+F4. IDAPro cheatsheet IDAPro 7.5 – document updated February 09, 2021. File Operations. Parse C header file.
Datarescue Interactive Disassembler (IDA) Pro Quick Reference Sheet (Open Subviews Names Shift+F4. IDAPro cheatsheet IDAPro 7.5 – document updated February 09, 2021. File Operations. Parse C header file.
Relative abundance of (note use of percents):
Ne-21 = 1%Calculating Average Atomic Mass Worksheet Pdf
Ne-22 = (9.5 − 1)% = 8.5%
